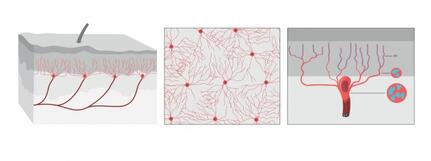
 The illustration shows what the organ looks like from the side, from above the skin. Illustrator: Mattias Karlén. Reprinted with permission from Abdo et al, Science, Vol. 365, Issue 6454, pp. 695-699 (2019). The illustration shows what the organ looks like from the side, from above the skin. Illustrator: Mattias Karlén. Reprinted with permission from Abdo et al, Science, Vol. 365, Issue 6454, pp. 695-699 (2019).
Pain is one of those feelings which if not all, most of us would never like to encounter and forget our previous encounters as well. However pain has been a necessary evil as it enables our bodies to recognize the occurrence of injuries or other problems which may not be always visible to our eyes, thus preventing further damage.
The International Association for the Study of Pain's widely used definition defines pain as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage". Before discovery of neurons responsible for pain by Charles Scott Sherrington in 1906 and the role of nociceptors, various theories were proposed to explain the origin of pain. Ancient Greeks including Hippocrates believed that it was due to an imbalance in vital fluids. Now a team of researchers from Karolinska Institutet have now discovered a new sensory receptor organ in the skin that is sensitive to hazardous environmental irritation. Conventionally pain has been thought to be initiated by activation of free nerve endings without end organs in the skin. In contrast to this paradigm, Abdo et al. discovered a previously unknown meshlike organ covering the skin that senses dangerous environmental stimuli. This organ is built from specialized glial cells with multiple long protrusions and which collectively go to make up a mesh-like organ within the epidermal-dermal border of skin. This organ is sensitive to painful mechanical damage such as pricks and pressure. The present study describes what the new pain-sensitive organ looks like, how it is organised together with pain-sensitive nerves in the skin and how activation of the organ results in electrical impulses in the nervous system that result in reflex reactions and an experience of pain. In their experiments, the researchers also blocked the organ and saw a resultant decreased ability to feel mechanical pain. "Our study shows that sensitivity to pain does not occur only in the skin's nerve fibres, but also in this recently-discovered pain-sensitive organ. The discovery changes our understanding of the cellular mechanisms of physical sensation and it may be of significance in the understanding of chronic pain," says Prof. Patrik Ernfors, professor at Karolinska Institutet's Department of Medical Biochemistry and Biophysics and chief investigator for the study. The research was carried out with financial assistance from ERC, the Swedish Research Council, the Knut and Alice Wallenberg Foundation and Welcome Trust.
References
1. Abdo H, Calvo-Enrique L, Martinez Lopez J, Song J, Zhang MD, Usoskin D, El Manira A, Adameyko I, Hjerling-Leffler J, Ernfors P. Specialized cutaneous Schwann cells initiate pain sensation. Science, 2019 DOI: 10.1126/science.aax6452 2. Image source: Karolinska Instituet 3. Source article: Karolinska Instituet
0 Comments
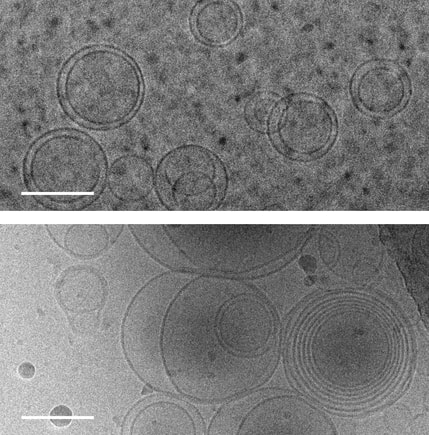 Images of membranes (circles) taken using transmission electron cryomicroscopy. Top: membranes in a solution that contains no amino acids. Bottom: membranes in a solution containing serine, an amino acid, which triggers membranes to form multiple layers of concentric membranes. Scale bars: 100 nanometers. Images of membranes (circles) taken using transmission electron cryomicroscopy. Top: membranes in a solution that contains no amino acids. Bottom: membranes in a solution containing serine, an amino acid, which triggers membranes to form multiple layers of concentric membranes. Scale bars: 100 nanometers.
The Blue planet of ours is still the only place in universe where we know life exists! However life's journey in this planet was no less than an adventurous journey when it started 4 billion years ago when the first cells formed within a primordial soup of complex, carbon-rich chemical compounds. The primitive life forms or coacervates faced a molecular conundrum as they needed charged ions to perform their basic functions however these ions could de-stabilize and disrupt the simple membranes that encapsulated the cells.
This puzzle has been solved by a team of researchers at University of Washington and their findings were published Aug. 12 in the Proceedings of the National Academy of Sciences. The team solved this puzzle using only molecules that would have been present on the early Earth. Using cell-sized, fluid-filled compartments surrounded by membranes made of fatty acid molecules, the team discovered that amino acids, the building blocks of proteins, can stabilize membranes against magnesium ions. Their results set the stage for the first cells to encode their genetic information in RNA, a molecule related to DNA that requires magnesium for its production, while maintaining the stability of the membrane. Beside explaining the mechanism how amino acid could stabilize the membranes in unfavorable condition they went beyond and demonstrated how the individual building blocks of cellular structures — membranes, proteins and RNA — could have co-localized within watery environments on the ancient Earth. “Cells are made up of very different types of structures with totally different types of building blocks, and it has never been clear why they would come together in a functional way,” said co-corresponding author Roy Black, a UW affiliate professor of chemistry and bioengineering. “The assumption was just that — somehow — they did come together.” Prof. Black teamed up with Sarah Keller, a UW professor of chemistry and an expert on membranes. Black had been inspired by the observation that fatty acid molecules can self-assemble to form membranes, and hypothesized that these membranes could act as a favorable surface to assemble the building blocks of RNA and proteins.“You can imagine different types of molecules moving within the primordial soup as fuzzy tennis balls and hard squash balls bouncing around in a big box that is being shaken,” said Keller, who is also co-corresponding author on the paper. “If you line one surface inside the box with Velcro, then only the tennis balls will stick to that surface, and they will end up close together. Roy had the insight that local concentrations of molecules could be enhanced by a similar mechanism.” The team previously had shown that the building blocks of RNA preferentially attach to fatty acid membranes and, surprisingly, also stabilize the fragile membranes against detrimental effects of salt, a common compound on Earth past and present. The team hypothesized that amino acids might also stabilize membranes. Using variety of experimental techniques — including light microscopy, electron microscopy and spectroscopy — to test how 10 different amino acids interacted with membranes. Their experiments revealed that certain amino acids bind to membranes and stabilize them. Some amino acids even triggered large structural changes in membranes, such as forming concentric spheres of membranes — much like layers of an onion. “Amino acids were not just protecting vesicles from disruption by magnesium ions, but they also created multilayered vesicles — like nested membranes,” said lead author Caitlin Cornell, a UW doctoral student in the Department of Chemistry. The researchers also discovered that amino acids stabilized membranes through changes in concentration. Some scientists have hypothesized that the first cells may have formed within shallow basins that went through cycles of high and low concentrations of amino acids as water evaporated and as new water washed in. The new findings that amino acids protect membranes — as well as prior results showing that RNA building blocks can play a similar role — indicate that membranes may have been a site for these precursor molecules to co-localize, providing a potential mechanism to explain what brought together the ingredients for life. Keller, Black and their team will turn their attention next to how co-localized building blocks did something even more remarkable: They bound to each other to form functional machines. "That is the next step,” said Prof. Black. The study was co-authored by Gary Drobny, UW professor of chemistry; Kelly Lee, UW associate professor of medicinal chemistry; UW postdoctoral researchers Mengjun Xue and Helen Litz in the Department of Chemistry, and James Williams in the Department of Medicinal Chemistry; UW graduate students Zachary Cohen in the Department of Chemistry and Alexander Mileant in the Biological Structure, Physics and Design Graduate Program; and UW undergraduate alumni Andrew Ramsay and Moshe Gordon. The research was funded by NASA, the National Institutes of Health and the National Science Foundation.
References
1. Original article byJames Urton UW news. Note: Content may be edited for style and length. 2. Image resource: Alex Mileant/Caitlin Cornell, University of Washington. 
In the whole world of love and affection, there is no love that beats the love of a mother for her children. Earlier exclusively attributed to humans only due to their improved cognitive functions and social structures, it is now a well documented fact that all mother's irrespective of their taxonomic position show a high level of caring for their children.
To understand it better now lets go back to our childhood, somehow our mothers always knew when we were hungry or when we experienced a agony. Mothers seem to have an instinct related to their children and if not all most of the time mothers are correct! How this happens? A recent discovery carried out by Ryoichi Teruyama and a team of student researchers at Louisiana State University (LA, USA) may have given scientists a more complete understanding of the mechanisms underpinning maternal instinct. The study has revealed differences in the distribution of neurons sensitive to oxytocin in female brains compared with male brains. Oxytocin is a neurotransmitter and hormone found in a variety of species. It is known as the love hormone, and plays a pivotal role in regulating maternal behavior, as well as being responsible for modulating a variety of social behaviors including: maternal care, sexual behavior in both sexes, bonding and anxiety. “Many researchers have attempted to investigate the difference between the oxytocin system in females versus males, but no one has successfully found conclusive evidence until now. Our discovery was a big surprise,” Teruyama commented. The team concentrated on the medial preoptic area (MPOA), an area of the brain that forms part of the essential neural circuit that regulates maternal behavior. Using oxytocin receptor (OXTR)-Venus knock-in mice, in which a yellow fluorescent protein is directly inserted into the locus where OXTR is usually located, scientists had the ability to image where OXTR was being expressed. Whilst there were no significant differences in the amount of OXTR cells located in the MPOA between male and females, it was demonstrated that neurons expressing OXTR were found in the anteroventral periventricular nucleus (AVPV) of the MPOA in females almost exclusively. When the female OXTR-Venus mice received ovariectomies, OXTR was no longer expressed in the AVPV. However, expression could be restored upon receiving estrogen replacement therapy. This finding denotes estrogen dependence and the female-specific nature of OXTR expression in the AVPV, indicating that these neurons play a role in the induction of maternal responses. Previous studies have shown a link between altered expression of OXTR and postpartum depression, which is associated with poor maternal health and substandard child development. This work has the potential to contribute to future treatments and pharmaceuticals for postpartum depression where the OXTR cells are targeted.
References
1. The original article published inBiotechniques. Note: Content edited for style and length. 2. Image courtesy: Pxhere.com. 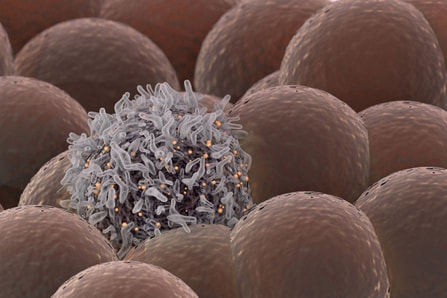 Stanford University researchers have discovered a new cellular signal that cancer cells seem to use to evade detection and destruction by the cells of the immune system, and macrophages in particular. Studies by the team of researchers in mice model paved a way for development of new therapeutic strategies. The scientists have shown that blocking this signal in mice implanted with human cancers allows immune cells to attack the cancers. Cancer cells have shown in earlier studies, that they choose to evade destruction by macrophages by overexpressing anti-phagocytic surface proteins called ‘don’t eat me’ signals such as CD471, programmed cell death ligand 1 (PD-L1) and the beta-2 microglobulin subunit of the major histocompatibility class I complex (B2M). Antibodies that block CD47 are in clinical trials. Cancer treatments that target PD-L1 are already being used in the clinics. The study lead by Amira Barkal, an MD-PhD student, (lead author). Irving Weissman, MD, professor of pathology and of developmental biology and director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine (senior author), showed that CD24 can be the dominant innate immune checkpoint in ovarian cancer and breast cancer, and is a promising target for cancer immunotherapy.Looking for additional signalsThe scientists began by looking for proteins that were produced more highly in cancers than in the tissues from which the cancers arose. “You know that if cancers are growing in the presence of macrophages, they must be making some signal that keeps those cells from attacking the cancer,” Barkal said. “You want to find those signals so you can disrupt them and unleash the full potential of the immune system to fight the cancer.” The search showed that many cancers produce an abundance of CD24 compared with normal cells and surrounding tissues. In further studies, the scientists showed that the macrophage cells that infiltrate the tumor can sense the CD24 signal through a receptor called SIGLEC-10. They also showed that if they mixed cancer cells from patients with macrophages in a dish, and then blocked the interaction between CD24 and SIGLEC-10, the macrophages would start gorging on cancer cells like they were at an all-you-can-eat buffet. “When we imaged the macrophages after treating the cancers with CD24 blockade, we could see that some of them were just stuffed with cancer cells,” Barkal said. Lastly, they implanted human breast cancer cells in mice. When CD24 signaling was blocked, the mice’s scavenger macrophages of the immune system attacked the cancer. Of particular interest was the discovery that ovarian and triple-negative breast cancer, both of which are very hard to treat, were highly affected by blocking the CD24 signaling. “This may be a vulnerability for those very dangerous cancers,” Barkal said. Complementary to CD47?The other interesting discovery was that CD24 signaling often seems to operate in a complementary way to CD47 signaling. Some cancers, like blood cancers, seem to be highly susceptible to CD47-signaling blockage, but not to CD24-signaling blockage, whereas in other cancers, like ovarian cancer, the opposite is true. This raises the hope that most cancers will be susceptible to attack by blocking one of these signals, and that cancers may be even more vulnerable when more than one “don’t eat me” signal is blocked. “There are probably many major and minor ‘don’t eat me’ signals, and CD24 seems to be one of the major ones,” Barkal said The researchers now hope that therapies to block CD24 signaling will follow in the footsteps of anti-CD47 therapies, being tested first for safety in preclinical trials, followed by safety and efficacy clinical trials in humans. For Weissman, the discovery of a second major “don’t eat me” signal validates a scientific approach that combines basic and clinical research. “These features of CD47 and CD24 were discovered by graduate students in MD-PhD programs at Stanford along with other fellows,” Weissman said. “These started as fundamental basic discoveries, but the connection to cancers and their escape from scavenger macrophages led the team to pursue preclinical tests of their potential. This shows that combining investigation and medical training can accelerate potential lifesaving discoveries.” References: 1. Original article: Stanford University of Medicine Note: The article has been edited for style and length 2. Journal article: Amira A. Barkal, Rachel E. Brewer, Maxim Markovic, Mark Kowarsky, Sammy A. Barkal, Balyn W. Zaro, Venkatesh Krishnan, Jason Hatakeyama, Oliver Dorigo, Layla J. Barkal, Irving L. Weissman. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature, 2019; DOI: 10.1038/s41586-019-1456-0 3. Image source: Stanford University of medince |
AuthorHello! My name is Arunabha Banerjee, and I am the mind behind Biologiks. Leaning new things and teaching biology are my hobbies and passion, it is a continuous journey, and I welcome you all to join with me Archives
June 2024
Categories
All
|

 RSS Feed
RSS Feed



