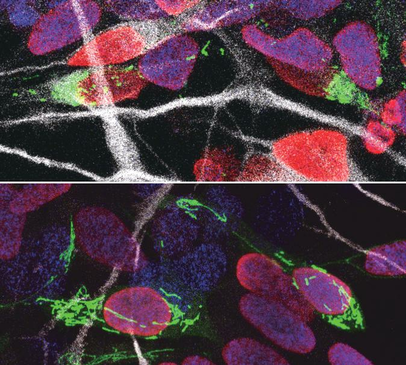
 Our brains are made up of billions of incredibly diverse neurons. They first arise in the developing brain when stem cells stop self-renewing and differentiate into a particular type of neuron. This process, called neurogenesis, is precisely regulated to give rise to the enormous complex structure that is our brain. It is thought that small differences in the way neural stem cells generate neurons are at the origin of the dramatic increase in the size and complexity of our brain. To gain insight in this complex process, prof. Pierre Vanderhaeghen (VIB-KU Leuven, ULB) and his colleagues examined the mitochondria, small organelles that provide energy in every cell in the body, including the developing brain. “Diseases caused by defects in mitochondria lead to developmental problems in many organs, in particular the brain,” explains Vanderhaeghen, a specialist in stem cell and developmental neurobiology. “We used to think that this was related to the crucial function of mitochondria to provide energy to the cells, but this is only part of the story: recent work in stem cells suggests that mitochondria have a direct influence on organ development. We have tested whether and how this could be the case in the brain.” Fission and fusion Together with his team, he explored whether and how mitochondrial remodeling is coupled with neuronal fate commitment during neurogenesis. “Mitochondria are highly dynamic organelles, that can join together (fusion) or split up (fission), and we know these dynamics are associated with fate changes in various types of stem cells,” says Vanderhaeghen. Ryohei Iwata, a postdoctoral researcher in the Vanderhaeghen lab, developed a new method to watch mitochondria in great detail as the neural stem cells are ‘caught in the act’ to become neurons. “We found that shortly after stem cells divide, the mitochondria in daughter cells destined to self-renew will fuse, while those in daughter cells that become neurons show high levels of fission instead,” says Ryohei Iwata. But this was not just a coincidence: indeed, the researchers could show that increased mitochondrial fission in fact promotes differentiation to a neuronal fate, while mitochondrial fusion after mitosis redirects daughter cells towards self-renewal. Time window So mitochondrial dynamics are important to become a neuron—but there is more. “We found that the influence of mitochondrial dynamics on cell fate choice is limited to a very specific time window, right after cell division,” says Pierre Casimir, a PhD student in Vanderhaeghen’s lab. “Interestingly, the restricted time window is twice as long in humans compared to mice.” “Previous findings were primarily focused on fate decision of neural stem cells before they divide, but our data reveal that cell fate can be influenced for a much longer period, even after neural stem cell division,” says Vanderhaeghen. This may have interesting implications in the emerging field of cell reprogramming, where scientists try to convert non-neuronal cells directly in neuronal cells for therapeutic purposes for instance. “Since this period of plasticity is much longer in human cells compared to mouse cells, it is tempting to speculate that it contributes to the increased self-renewal capacity of human progenitor cells, and thus to the uniquely developed brain and cognitive abilities of our species. It is fascinating to think that mitochondria, small organelles that have evolved in cells more than a billion years ago, might have contributed to the recent evolution of the human brain.” Story Source:
News article from The Flanders Institute for Biotechnology. Note: Content edited for style. Journal Reference: Ryohei Iwata, Pierre Casimir, Pierre Vanderhaeghen. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science, 2020 DOI: 10.1126/science.aba9760
0 Comments
 Pluripotent stem cells have the ability to self-renew and give rise to all other types of cells and organs in the body. The developmental patterns and characteristics are controlled by a network of regulatory genes and molecules, but little is known about how this network has evolved across mammals. The recent study by Kyoto University researchers across 48 mammalian species published in the journal Genome Biology and Evolution. The study group observed that the genes regulating pluripotent stem cells in mammals are surprisingly similar. In the study conducted by Ken-ichiro Kamei of Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS), with Miho Murayama and Yoshinori Endo of the Wildlife Research Center, compared 134 gene sets belonging to the pluripotency gene regulatory networks of 48 mammalian species. They found that this network has been highly conserved across species, meaning genetic sequences have remained relatively unchanged over the course of evolution. This high degree of conservation explains why human genetic sequences can reprogram other mammalian tissue cells to turn into pluripotent stem cells. However, since it is also evident that the regulating networks differ across mammals, there might be more efficient combinations of reprogramming factors for each species. Improving techniques for deriving induced pluripotent stem (iPS) cells from mammalian cells, including those from endangered species, could provide a big boost to research and conservation. “We have been trying to generate induced pluripotent stem cells from various mammalian species, such as the endangered Grévy’s zebra and the bottlenose dolphin,” says Kamei. Interestingly, the team found relatively high evolutionary changes in genes just downstream of one of the core gene regulatory networks. “This could indicate that mammalian pluripotent stem cells have diversified more than we thought,” says Inoue-Murayama. The differences between gene regulatory networks in mammalian pluripotent stem cells might also be associated with unique adaptions. For example, the naked mole rat has been positively selected for a pluripotency regulatory gene that could be involved in giving it its extraordinary longevity and cancer resistance. The gene might also be involved in the development of the extremely sensitive hairs that help them navigate underground. The researchers also found evidence of positive selection for certain pluripotency gene regulatory network genes involved in the adaptation of large animals, such as the minke whale, the African elephant and the flying fox, to their environments. Surprisingly, these same genes are associated with cancer in other mammals. Since these large animals are known for being relatively resistant to cancer, the researchers suggest that the adaptive alterations these genes underwent in these animals somehow also changed some of their functions, thus giving this group a degree of cancer resistance. The researchers say the study is among the first to compare the pluripotency gene regulatory networks across major taxa, and could be applicable to evolutional biology studies and for facilitating and improving the generation of induced pluripotent stem cells from new species. Story Source:
Kyoto University. Content edited for style and length Journal Reference: Yoshinori Endo, Ken-ichiro Kamei, Miho Inoue-Murayama. Genetic signatures of evolution of the pluripotency gene regulating network across mammals. Genome Biology and Evolution, 2020; DOI: 10.1093/gbe/evaa169  Recent findings by researchers at Yale points towards a host of genetic risk factors that explains susceptibility to the debilitating symptoms of post-traumatic stress disorder (PTSD) in veterans. The Yale-led study published on Sept. 30 in the journal Biological Psychiatry has now identified a social factor that can mitigate these genetic risks: the ability to form loving and trusting relationships with others. The study is one of the first to explore the role of nurture as well as nature in its investigation of the biological basis of PTSD. “We exist in a context. We are more than our genes,” said Yale’s Robert H. Pietrzak, associate professor of psychiatry and public health, and senior author of the study. Pietrzak is also director of the Translational Psychiatric Epidemiology Laboratory of the U.S. Department of Veterans Affairs National Center for PTSD. Like many genetic studies on mental disorders such as depression, anxiety, and schizophrenia, PTSD studies have revealed numerous genetic risk factors that contribute to the severity of the disorder. For instance, a previous study of more than 165,000 U.S. military veterans led by Yale’s Joel Gelernter, the Foundations Fund Professor of Psychiatry and professor of genetics and of neuroscience, found variants in eight separate regions of the genome that help predict who is most likely to experience the repeated disturbing memories and flashbacks that are hallmark symptoms of PTSD. In the new study, Pietrzak, Gelernter, and colleagues looked at psychological as well as genetic data collected from the National Health and Resilience in Veterans Study, a national sample of U.S. military veterans supported by the National Center for PTSD. The researchers specifically focused on a measure of attachment style — the ability or inability to form meaningful relations with others — as a potential moderator of genetic risk for PTSD symptoms. Individuals with a secure attachment style perceive relationships as stable, feel that they are worthy of love and trust, and are able to solicit help from others. Those with an insecure attachment style report an aversion to or anxiety about intimacy with others, and have difficulty asking for help from others. They found that the ability to form secure attachments essentially neutralized the collective effects of genetic risk for PTSD symptoms. The impact was particularly pronounced in a variant of the IGSF11 gene, which has been linked to synaptic plasticity or the ability of the brain to form new connections between brain cells. Pietrzak noted that deficits in synaptic plasticity have also been linked to PTSD, depression, and anxiety, among other mental disorders. The findings illustrate the importance of integrating environmental and social as well as genetic factors in the study of PTSD and other mental disorders, the authors said. “Social environmental factors are critical to informing risk for PTSD and should be considered as potential moderators of genetic effects,” he said. “The ability to form secure attachments is one of the strongest protective factors for PTSD and related disorders.” The attachment styles may moderate polygenic risk for PTSD symptoms, along with the effects of a novel locus implicated in synaptic transmission and plasticity which may serve as a possible biological mediator of this association. These findings may help inform interpersonally-oriented treatments for PTSD for individuals with high polygenic risk for this disorder and will help predict who is at greater risk of experiencing severe symptoms of PTSD, the study also suggest that psychological treatments targeting interpersonal relationships may help mitigate PTSD symptoms in veterans with elevated genetic risk for this disorder . Amanda Tamman, formerly of Yale and now a Ph.D. student in clinical psychology at St. John’s University, is first author of the paper. Story Source:
Yale News bulletin written by By Bill Hathaway. Article modified for style and clarity, Image source: Wikimedia Commons Journal Article: Amanda J.F. Tamman, Frank R. Wendt, Gita A. Pathak, John H. Krystal, Janitza L. Montalvo-Ortiz, Steven M. Southwick, Lauren M. Sippel, Joel Gelernter, Renato Polimanti, Robert H. Pietrzak. Attachment style moderates polygenic risk for posttraumatic stress in United States military veterans: Results from the National Health and Resilience in Veterans Study. Biological Psychiatry, 2020; DOI: 10.1016/j.biopsych.2020.09.018 |
AuthorHello! My name is Arunabha Banerjee, and I am the mind behind Biologiks. Leaning new things and teaching biology are my hobbies and passion, it is a continuous journey, and I welcome you all to join with me Archives
June 2024
Categories
All
|

 RSS Feed
RSS Feed



