|
Scientists at Tokyo Institute of Technology have imaged live T cells to reveal the role of CLIP-170 in T-cell activation, a critical process in the immune response.
When bacteria or viruses enter the body, proteins on their surfaces are recognized and processed to activate T cells, white blood cells with critical roles in fighting infections. During T-cell activation, a molecular complex known as the microtubule-organizing center (MTOC) moves to a central location on the surface of the T-cell. Microtubules have several important functions, including determining cell shape and cell division. Thus, MTOC repositioning plays a critical role in the immune response initiated by activated T cells.
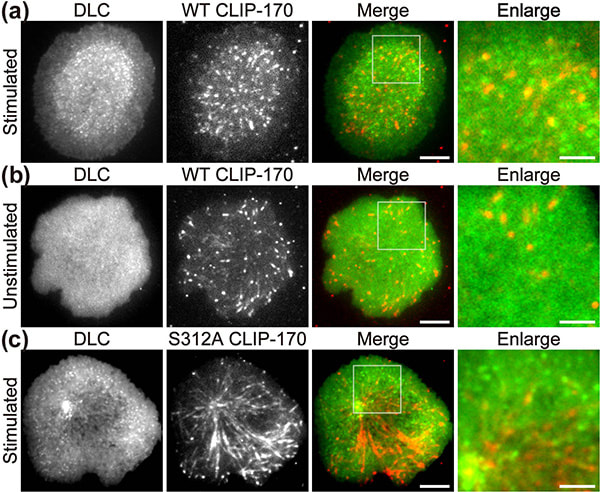
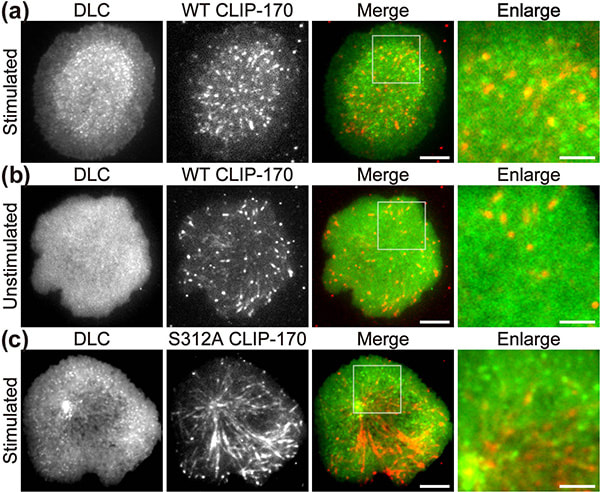
In a recent publication in Scientific Reports, the first authors Lim Wei Ming and Yuma Ito, along with their colleagues at Tokyo Institute of Technology (Tokyo Tech), provide compelling evidence that a key protein responsible for the relocation of the MTOC in activated T cells is a molecule known as CLIP-170, a microtubule-binding protein. The researchers used live-cell imaging to uncover the mechanism of MTOC relocation. “The use of dual-color fluorescence microscopic imaging of live T cells allowed us to visualize and quantify the molecular interactions and dynamics of proteins during MTOC repositioning,” notes Dr. Sakata-Sogawa. This technque allowed them to confirm that phosphorylation of CLIP-170 is involved in movement of the MTOC to the center of the contacted cell surface (Fig. 1); the findings were confirmed using both cells with phosphodeficient CLIP-170 mutant and cells in which AMPK, the molecule that phosphorylates and activates CLIP-170, was impaired. Further imaging showed that CLIP-170 is essential for directing dynein, a motor protein, to the plus ends of microtubules and for anchoring dynein in the center of the cell surface (Fig. 2). Dynein then pulls on the microtubules to reposition the MTOC to its new location in the center. “These findings shed new light on microtubule binding proteins and microtubule dynamics,” explains Dr. Tokunaga. Such research is critical, as a deeper understanding of T cell activation in the immune response, and could lead to the development of safer methods for cancer immunotherapy because presentation of CTLA-4, which is found by a 2018 Novel Prize laureate and used as a target of the therapy, is also regulated by MTOC reposition
Figure 1. CLIP-170 phosphorylation regulates MTOC repositioning and full activation of T cells.
Fluorescence live-cell imaging of the wild-type CLIP-170-TagRFP-T (a,b) or a phosphodeficient S312A mutant CLIP-170-TagREP-T (c) and dynein light chain (DLC)-mEGFP co-expressed in T cells. Increased dynein relocation to the center, which is responsible for MTOC repositioning, requires both stimulation and CLIP-170 phosphorylation. The boxed regions in the merged images are enlarged (right). Scale bars: 5 μm (left, 2nd left, merged) and 2 μm (right)
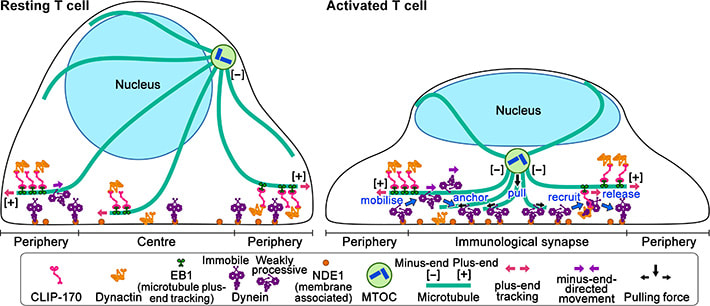
Figure 2: Schematic model for the keyrole of CLIP-170 in MTOC repositioning during T cell activation by regulating dynein relocation.
In resting T cells, the majority of dynein is immobile on the contacted cell surface and is located at the periphery region. T cell stimulation increases the fraction of dynein undergoing minus-end-directed motility (“mobilise”), which is a “weakly processive” state. Then, the dynein is anchored to the surface (“anchor”). Alongside this, stimulation induces some fraction of dynein to colocalize with CLIP-170 and dynactin and follow plus-end tracking (“recruit”). After tracking of one or two micrometers, the dynein is released from the complex and anchored (“release”). As a result, dynein relocation increases to the center region of the contact surface, the immunological synapse, where “anchored” dynein molecules are immobile and or weakly processive at a velocity in good agreement with the velocity of MTOC repositioning. “Anchored” and weakly processive dynein pulls the microtubules and the MTOC (“pull”), which causes MTOC repositioning near the immunological synapse and full activation of T cells. Phosphorylation of CLIP-170 is essential for dynein recruitment to the plus-end and for dynein relocation. ***************************************************************** References 1. Original article source: School of Life Science and Technology, Tokyo Institute of Technology 2. Research Paper: Wei Ming Lim, Yuma Ito, Kumiko Sakata-Sogawa* & Makio Tokunaga* CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface Nature Scientific Reports DOI : 10.1038/s41598-018-35593-z 3. Image source: School of Life Science and Technology, Tokyo Institute of Technology
0 Comments
New research demonstrates antiparasitic drug turns lethal for malaria-carrying mosquitoes showing promising future for the drug. Preliminary analysis showed that the drug reduced malaria cases in children under 5 by 16%. The trends are encouraging for the drug and it might become part of national malaria control programs.
Ivermectin a well known antiparasitic drug for treatment of a wide range of parasite infestations from head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, and lymphatic filariasis, may have another hidden benefit.
Mosquitoes of the genus Anopheles and only females of the species are capable of transmitting the malaria pathogen a protozoa, of genus Plasmodium, which undergoes a series of infection steps before arriving at the mosquito’s salivary gland, from where it ultimately gets transmitted to the human host during blood meal. Malaria has been a well known killer in the tropical regions of the globe killing more peoples combined who died from outer causes. The data for malaria infection and transmission is staggering as each year, the disease infects more than 200 million people, causing 429,000 deaths — and the situation seems to get worse as despite spending billions on malaria eradication programs, we seem to have reached a plateau. Meanwhile mosquitoes are becoming increasingly resistant to insecticides, which is forcing researchers to think of all sorts of new solutions like a malaria vaccine, genetically engineering mosquitoes so they wipe themselves out and many more. Previous studies have found that malaria-carrying mosquitoes would die after sucking the blood from individuals who had taken ivermectin, researchers have known for decades that the drug also kills insects if they ingest it. Brian Foy, a medical entomologist at Colorado State University, Fort Collins, believes that makes it a prime candidate in the fight against malaria. If enough people in an area have ivermectin in their blood, says Foy, some of the female mosquitoes that bite them will die, whereas others will be too weakened to pass on the malaria parasite. Foy has shown in lab studies that the approach holds promise, and co-founded a research network last year to study the concept further, to show that ivermectin actually has an impact on malaria in the field, Foy teamed up with Roch Dabiré, a researcher at the Institute of Health Studies in Bobo-Dioulasso, Burkina Faso. The scientists went to eight villages near the town of Diébougou, in the southwest of the country. At the start of the trial, in July, the population of all villages received one dose of ivermectin and another drug, albendazole; this standard combination is given twice yearly around Burkina Faso to control elephantiasis and soil-transmitted worms. In four of the villages, this was followed by ivermectin tablets every 3 weeks for the entire population except pregnant women and children under 90 centimeters tall, who may be at higher risk of side effects. The four other villages served as controls; they received no drugs after the first dose. The trial is still ongoing and will conclude in November. But an interim analysis presented today by Foy and Dabiré at the annual meeting of the American Society of Tropical Medicine and Hygiene suggests that the drug is already having an impact. Among children under the age of 5—the group at the highest risk of severe disease and death from malaria—there were 16% fewer cases in the villages that received ivermectin at 3-week intervals. That translates to 94 cases averted so far this season in the four villages. The full results will take some time to analyze, and the study will need to be repeated at a larger scale to see if the findings hold up, Dabiré says. If they do, ivermectin could be another weapon in the antimalaria arsenal, Foy says. He adds that it wouldn’t replace other measures, such as insecticide-treated bed nets. It’s an interesting approach that should be explored further, says Michel Boussinesq, who studies ivermectin at the Institute of Research for Development in Montpellier, France. But the need to give ivermectin every 3 weeks could be a logistical problem, he says. Boussinesq and his colleagues are working on an ivermectin implant for animals that instead releases the drug slowly and offers long-term protection. Such implants aren’t likely to be acceptable for use in humans, Foy says—but he points out that ivermectin would only be given during the rainy season, when malaria mosquitoes are active. The season lasts about 6 months in the region where the study took place and even less than that farther north, in the Sahel region. “I think that’s feasible,” Foy says. Willem Takken, a medical entomologist at Wageningen University and Research Centre in the Netherlands, sees another, fundamental problem: Mosquitoes have developed resistance against almost any chemical that humans have thrown at them. He says that’s bound to happen with ivermectin, too. That’s why, despite the encouraging data, “I find it hard to get enthusiastic about this,” Takken says. He believes that nonchemical approaches, such as mosquito traps or bacteria that render mosquitoes unable to transmit pathogens, hold more long-term promise. ************************************************************ References:
Recent study by a group of scientists from Intermountain Healthcare Heart Institute in Salt Lake City, identified eight new gene mutations that may contribute to idiopathic dilated cardiomyopathy, a form of heart disease not caused by known external influences.
In a new study from the Intermountain Healthcare Heart Institute in Salt Lake City, led by Dr. Jeffrey L. Anderson, MD, the researchers have identified eight new gene mutations that may cause or contribute to idiopathic dilated cardiomyopathy, a form of heart disease not caused by known external influences, such as high blood pressure, obesity, smoking, or diseased coronary arteries. The study observed that for at least 40 percent of the enrolled patients, the disease had an underlying genetic cause that leads to the muscle in the major pumping chamber of the heart (left ventricle) being too weak and thin to function properly, causing heart failure.
“Although many mutations contributing to non-ischemic dilated cardiomyopathy have been identified, there remains a large gap in our knowledge of its heritability. The more we can learn about what’s causing the condition, the better we can identify and treat it,” said Jeffrey L. Anderson, MD, principal investigator of the study, and a researcher at the Intermountain Healthcare Heart Institute. “If it’s passed on in families, we’ll be able to identify those who might be at risk for developing heart disease and work to prevent it, diagnose it, and begin treatment earlier.” The study team is going to present the findings from the study at the American College of Cardiology’s Annual Scientific Session in New Orleans on March 18, 2019. A quarter to one-third of idiopathic dilated cardiomyopathy patients will need a mechanical support device, a heart transplant, or will die within five years, Dr. Anderson noted, so this is a very serious condition. In the study, researchers looked at genetic samples of 231 patients with idiopathic dilated cardiomyopathy, evaluated in an Intermountain Medical Center Specialty Clinic who volunteered to enter blood samples into the Intermountain Healthcare INSPIRE Registry and DNA Bank, which is the system’s collection of biological samples, clinical information, and laboratory data from consenting patients who are diagnosed with any of a number of healthcare-related conditions. In collaboration with Intermountain’s Precision Genomics laboratory, researchers sequenced patients’ DNA, focusing on the TITIN (TNN) gene, which codes the body’s largest protein. “That protein acts as a spring in your heart muscle,” said Dr. Anderson. “It enhances the passive elasticity of the muscle and also limits how much you can stretch it.” Previous studies have already observed variants of TTN in patients with idiopathic dilated cardiomyopathy, but the story has been incomplete. Now, in this new study, Intermountain researchers identified 24 patients with TTN variants, and eight of those variants hadn’t been seen or documented before. They also confirmed the presence of seven variants that had been discovered and reported previously. The new variants all are of the “truncating” variety, that is, they lead to a shortening of the protein and in doing so it is predicted to cause the protein to malfunction in its role of maintaining the integrity of heart muscle function. These new variants, Dr. Anderson said, still will require functional testing and clinical validation, but they likely will lead to further expansion of the known spectrum of genes that predispose to idiopathic dilated cardiomyopathy. The addition of these variants to the current list of known pathological heart muscle protein mutations will help to close the still large gap in our knowledge of the heritability of heart muscle disease and in doing so can lead to earlier diagnosis and more effective prevention and treatment. The study was funded by the Intermountain Foundation and an in-kind grant from Intermountain Precision Genomics. ************************************** References
The compounds in frying oils that are repeatedly reheated to high temperatures may trigger cell proliferation and metastases in breast tumors, scientists in food science and human nutrition at the University of Illinois found in a new study of mice model. A common scene we observe in our roadside food stalls is that the cooking oil used for frying eatables is often reused, the prime reason being cost effectiveness, however its risks are often discussed in closed circles but no concrete steps are ever taken. However now a consciousness is seen to be arising and some regulatory bodies like FSSAI of India issued directive against reusing cooking oil.
In the present study published in the journal of Cancer Prevention Research the scientists observed that, thermally abused frying oil ( cooking oil that has been repeatedly reheated to high temperatures) may act as a toxicological trigger that promotes tumor cell proliferation, metastases and changes in lipid metabolism. The study conducted in mice also suggests that consuming the chemical compounds found in thermally abused cooking oil may trigger genetic changes that promote the progression of late-stage breast cancer. In this study the mice were divided into two groups consuming a low-fat diet for one week, one group of the mice was fed unheated fresh soybean oil, while another group consumed thermally abused oil for the next 16 weeks. Soybean oil was used in the study because of its common use by the food service industry in deep frying. The team of research scientists simulated late-stage breast cancer by injecting 4T1 breast cancer cells into a tibia of each mouse. The 4T1 cells are an aggressive form of the disease that can spontaneously metastasize to multiple distant sites in the body, including the lungs, liver and lymph nodes, according to the study. Twenty days after inoculation with the tumor cells, the primary tumors in the tibias of the mice that consumed the thermally abused oil had more than four times as much metastatic growth as the mice that consumed the fresh soybean oil. And when the researchers examined the animals’ lungs, they found more metastases among those that consumed the thermally abused oil. “There were twice as many tumors in the lung, and they were more aggressive and invasive,” said William G. Helferich, a professor of food science and human nutrition, who led the research. Food chemistry professor Nicki J. Engeseth, the acting head of the department, co-wrote the paper. Graduate student Ashley W. Oyirifi and U. of I. alumnus Anthony Cam were the lead authors. “I just assumed these nodules in the lungs were little clones – but they weren’t. They’d undergone transformation to become more aggressive. The metastases in the fresh-oil group were there, but they weren’t as invasive or aggressive, and the proliferation wasn’t as extensive,” Helferich said. In examining both groups of mice, the scientists found that the metastatic lung tumors in those that consumed thermally abused frying oil expressed significantly more of a key protein, Ki-67, which is associated strictly with cell proliferation. Gene expression in these animals’ livers was altered as well. When the researchers conducted RNA sequencing analysis, they found 455 genes in which expression was at least two times greater – or, conversely, two times lower – than in mice that consumed the fresh oil. The altered gene pathways were associated with oxidative stress and the metabolism of foreign substances, Oyirifi said. When oil is repeatedly reused, triglycerides are broken apart, oxidizing free fatty acids and releasing acrolein, a toxic chemical that has carcinogenic properties. Scientists have long known that thermally abused oil contains acrolein, and studies have linked the lipid peroxides in it with a variety of health problems, including atherosclerosis and heart disease. As the oil degrades, polymer molecules also accumulate, raising nutritional and toxicological concerns, Engeseth said. Countries in Europe and elsewhere regulate the amount of polar materials in frying oil, which are chemically altered triglycerides and fatty acids that are used as chemical markers of oils’ decomposition. Typically, these standards permit restaurants to use oil containing up to 24-27 percent polar material. By contrast, the thermally abused oil used in the current study contained about 15 percent polar material, while fresh oil contains 2-4 percent or less, Helferich said. “Because there are no regulations in the U.S., it’s really difficult for us to evaluate what’s out there,” Engeseth said. “But the important thing is, the food that’s fried in these oils sucks up quite a bit of oil. Even though we’re not consuming the oil directly, we’re consuming oil that’s brought into the food during the frying process.” Breast cancer survivors’ biggest fear is recurrence, and the majority of these survivors have dormant tumor cells circulating in their blood, Helferich said. “What wakes those cells up is anybody’s guess, but I’m convinced that diet activates them and creates an environment in different tissues that’s more fertile for them to grow,” he said. “Many cancer biologists are trying to understand what’s happening at metastatic sites to prime them for tumor growth,” Oyirifi said. “We’re trying to add to this conversation and help people understand that it might not be just some inherent biological mechanism but a lifestyle factor. If diet provides an opportunity to reduce breast cancer survivors’ risk, it offers them agency over their own health.” Additional co-authors on the study were Urszula T. Iwaniec and Russell T. Turner, both of Oregon State University; and Fureya (Yunxian) Liu, a then-graduate student at the U. of I. The National Center for Complementary and Integrative Health, the Office of Dietary Supplements, the National Cancer Institute and the National Institute of Environmental Health Sciences funded the research. ************************************************************ Note: The article is edited for style and length References
The 28,000-year-old remains of a woolly mammoth, named ‘Yuka’, were found in Siberian permafrost. The study group recovered the less-damaged nucleus-like structures from the remains and visualised their dynamics in living mouse oocytes after nuclear transfer (aka cloning). In the reconstructed oocytes, the mammoth nuclei showed the spindle assembly, histone incorporation and partial nuclear formation; however, the full activation of nuclei for cleavage was not confirmed. The scientists hope their work provides a platform to evaluate the biological activities of nuclei in extinct animal species. Wolly mammoth an ice age giant often found plodding in animated movies like The ice age, actually died out just over 4,000 years ago, and climate change was one of the most probable reason for their death apart from alleged viral infections but could the prehistoric giants be soon back to life? Probably not, however the work by the team led by Dr. Akira Iritani from Institute of Advanced Technology, Kindai University, Wakayama,Japan provides a platform to evaluate the biological activities of nuclei in extinct animal species.
Their results published in the journal Nature Scientific reports indicates that “a part of mammoth nuclei possesses the potential for nuclear reconstitution, while observing possible signs of repair to damaged mammoth DNA.” However despite the successes, the scientists did not observe the further cell division necessary to create a viable egg, “possibly due to the extensive DNA damage in the transferred nuclei”. Researcher Kei Miyamoto, one of the study’s authors told Japan’s Nikkei news outlet. “We want to move our study forward to the stage of cell division,” he added, but acknowledged “we still have a long way to go”. The samples of woolly mammoth (named Yuka) used in the present study was found in Siberian permafrost in 2010. The animal, is beleived to be of about seven-years-old at the time of death and it is one of the best preserved mammoths known to science. Dr. Akira’s team extracted tissue samples from the animal’s bone marrow and muscle. It is worth mentioning that most mammoth populations died out between 14,000 and 10,000 years ago. The last mainland population existed in the Kyttyk peninsula of Siberia until 9,650 years ago. But the species is observed to be surviving for another 5,000 years on Siberian islands, which became cut off from the mainland by retreating ice following the last ice age. The last known population remained on Wrangel Island in the Arctic Ocean until 4,000 years ago – well beyond the dawn of human civilisation, but finally becoming extinct around the time of the construction of the pyramids of Giza in Egypt. The real cause for their extinction is still a issue where no scientific consensus has been reached, climate change leading to habitat destruction and hunting by humans are commonly discussed theories and works like that of Dr. Akira might shine some light on the other aspects of their extinction. **************************************************************** Note: The article has been edited for style and length References:
If you have a sweet tooth and often crave for a cola, I think you need to pay attention what lies ahead. A cola or a carbonated drink is usually our resort to cool off during a hot summer day, however its a well known fact that it packs a lot of false calories and people on diet strictly avoid drinking cola.
The main reason cola is high in calories is a common sweetening agent present in all processed foods, High-fructose corn syrup (HFCS), also known as glucose-fructose, isoglucose and glucose-fructose syrup, is a sweetener made from corn starch. Although extensively used in almost all processed foods HFCS has been found to be closely associated with Obesity, Diabetes. However the present study led by researchers at Baylor College of Medicine and Weill Cornell Medicine published in Science, showed that consuming a daily modest amount of high-fructose corn syrup – the equivalent of people drinking about 12 ounces of a sugar-sweetened beverage daily – accelerates the growth of intestinal tumors in mouse models of the disease, independently of obesity. The team also discovered the mechanism by which the consumption of sugary drinks can directly feed cancer growth, suggesting potential novel therapeutic strategies.“An increasing number of observational studies have raised awareness of the association between consuming sugary drinks, obesity and the risk of colorectal cancer,” said co-corresponding author Dr. Jihye Yun, assistant professor of molecular and human genetics at Baylor. “The current thought is that sugar is harmful to our health mainly because consuming too much can lead to obesity. We know that obesity increases the risk of many types of cancer including colorectal cancer; however, we were uncertain whether a direct and causal link existed between sugar consumption and cancer. Therefore, I decided to address this important question when I was a postdoc in the Dr. Lewis Cantley lab at Weill Cornell Medicine. First, Yun and her colleagues generated a mouse model of early-stage colon cancer where APC gene is deleted. “APC is a gatekeeper in colorectal cancer. Deleting this protein is like removing the breaks of a car. Without it, normal intestinal cells neither stop growing nor die, forming early stage tumors called polyps. More than 90 percent of colorectal cancer patients have this type of APC mutation,” Yun said. Using this mouse model of the disease, the team tested the effect of consuming sugar-sweetened water on tumor development. The sweetened water was 25 percent high-fructose corn syrup, which is the main sweetener of sugary drinks people consume. High-fructose corn syrup consists of glucose and fructose at a 45:55 ratio. When the researchers provided the sugary drink in the water bottle for the APC-model mice to drink at their will, mice rapidly gained weight in a month. To prevent the mice from being obese and mimic humans’ daily consumption of one can of soda, the researchers gave the mice a moderate amount of sugary water orally with a special syringe once a day. After two months, the APC-model mice receiving sugary water did not become obese, but developed tumors that were larger and of higher-grade than those in model mice treated with regular water. “These results suggest that when the animals have early stage of tumors in the intestines – which can occur in many young adult humans by chance and without notice – consuming even modest amounts of high-fructose corn syrup in liquid form can boost tumor growth and progression independently of obesity,” Yun said. “Further research is needed to translate these discovery to people; however, our findings in animal models suggest that chronic consumption of sugary drinks can shorten the time it takes cancer to develop. In humans, it usually takes 20 to 30 years for colorectal cancer to grow from early stage benign tumors to aggressive cancers.” “This observation in animal models might explain why increased consumption of sweet drinks and other foods with high sugar content over the past 30 years is correlating with an increase in colorectal cancers in 25 to 50-year-olds in the United States,” said Cantley, co-corresponding author, former mentor of Yun and professor of cancer biology in medicine and director of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. The team then investigated the mechanism by which this sugar promoted tumor growth. They discovered that the APC-model mice receiving modest high-fructose corn syrup had high amounts of fructose in their colons. “We observed that sugary drinks increased the levels of fructose and glucose in the colon and blood, respectively and that tumors could efficiently take up both fructose and glucose via different routes.” Using cutting-edge technologies to trace the fate of glucose and fructose in tumor tissues, the team showed that fructose was first chemically changed and this process then enabled it to efficiently promote the production of fatty acids, which ultimately contribute to tumor growth. “Most previous studies used either glucose or fructose alone to study the effect of sugar in animals or cell lines. We thought that this approach did not reflect how people actually consume sugary drinks because neither drinks nor foods have only glucose or fructose. They have both glucose and fructose together in similar amounts,” Yun said. “Our findings suggest that the role of fructose in tumors is to enhance glucose’s role of directing fatty acids synthesis. The resulting abundance of fatty acids can be potentially used by cancer cells to form cellular membranes and signaling molecules, to grow or to influence inflammation.” To determine whether fructose metabolism or increased fatty acid production was responsible for sugar-induced tumor growth, the researchers modified APC-model mice to lack genes coding for enzymes involved in either fructose metabolism or fatty acid synthesis. One group of APC-model mice lacked an enzyme KHK, which is involved in fructose metabolism, and another group lacked enzyme FASN, which participates in fatty acid synthesis. They found that mice lacking either of these genes did not develop larger tumors, unlike APC-model mice, when fed the same modest amounts of high-fructose corn syrup. “This study revealed the surprising result that colorectal cancers utilize high-fructose corn syrup, the major ingredient in most sugary sodas and many other processed foods, as a fuel to increase rates of tumor growth,” Cantley said. “While many studies have correlated increased rates of colorectal cancer with diet, this study shows a direct molecular mechanism for the correlation between consumption of sugar and colorectal cancer.” “Our findings also open new possibilities for treatment,” Yun said. “Unlike glucose, fructose is not essential for the survival and growth of normal cells, which suggests that therapies targeting fructose metabolism are worth exploring. Alternatively, avoiding consuming sugary drinks as much as possible instead of relying on drugs would significantly reduce the availability of sugar in the colon.” While further studies in humans are necessary, Yun and colleagues hope this research will help to raise public awareness about the potentially harmful consequences consuming sugary drinks has on human health and contribute to reducing the risk and mortality of colorectal cancer worldwide. Other contributors to this work include Drs. Sukjin Yang, Yumei Wang and Justin Van Riper with Baylor, Marcus Goncalves (lead author), Changyuan Lu, Jordan Trautner, Travis Hartman, Seo-Kyoung Hwang, Charles Murphy, Roxanne Morris, Sam Taylor, Quiying Chen, Steven Gross and Kyu Rhee, all with Weill Cornell Medicine, Chantal Pauli with the University Hospital Zurich, Kaitlyn Bosch with the Icahn School of Medicine at Mount Sinai, H Carl Lekaye with Memorial Sloan Kettering Cancer Center, Jatin Roper with Duke University and Young Kim with Chonnam National University. This study was supported by the National Institutes of Health, Stand Up 2 Cancer, the Cancer Prevention and Research Institute of Texas and the National Cancer Institute. Note: Content may be edited for style and length. ******************************************************************** References: 1. Original article can be accessed here : https://www.bcm.edu/news/molecular-and-human-genetics/high-fructose-corn-syrup-intestinal-tumors 2. Journal Reference: Marcus D. Goncalves, Changyuan Lu, et al, High-fructose corn syrup enhances intestinal tumor growth in mice. Science, 2019; 363 (6433): 1345-1349 DOI: 10.1126/science.aat8515 3. Image source: For representation only, https://goo.gl/images/s4dkKy DNA computing, a soon to be reality for tackling the next level of computational challenges3/22/2019 Computer scientists at Caltech have designed DNA molecules that can carry out reprogrammable computations, for the first time creating so-called algorithmic self-assembly in which the same "hardware" can be configured to run different "softwares" I remember during my graduation days early 2005-06 I could lay my hands on a science magazine called junior science refresher, although the magazine was not a top notch but nevertheless it was the only science news available in my state at that time. During my course of reading the magazine I came across the term "DNA computing" which fascinated me as a life-science student that one day the lving molecule could replace our silicon based computers. Now this recent development by Caltech caught my eye so thought of sharing the information with my readers and friends. Here is the original new byte from caltech.
In a paper publishing in Nature on March 21, a team headed by Caltech's Erik Winfree (PhD '98), professor of computer science, computation and neural systems, and bioengineering, showed how the DNA computations could execute six-bit algorithms that perform simple tasks. The system is analogous to a computer, but instead of using transistors and diodes, it uses molecules to represent a six-bit binary number (for example, 011001) as input, during computation, and as output. One such algorithm determines whether the number of 1-bits in the input is odd or even, (the example above would be odd, since it has three 1-bits); while another determines whether the input is a palindrome; and yet another generates random numbers. "Think of them as nano apps," says Damien Woods, professor of computer science at Maynooth University near Dublin, Ireland, and one of two lead authors of the study. "The ability to run any type of software program without having to change the hardware is what allowed computers to become so useful. We are implementing that idea in molecules, essentially embedding an algorithm within chemistry to control chemical processes." The system works by self-assembly: small, specially designed DNA strands stick together to build a logic circuit while simultaneously executing the circuit algorithm. Starting with the original six bits that represent the input, the system adds row after row of molecules—progressively running the algorithm. Modern digital electronic computers use electricity flowing through circuits to manipulate information; here, the rows of DNA strands sticking together perform the computation. The end result is a test tube filled with billions of completed algorithms, each one resembling a knitted scarf of DNA, representing a readout of the computation. The pattern on each "scarf" gives you the solution to the algorithm that you were running. The system can be reprogrammed to run a different algorithm by simply selecting a different subset of strands from the roughly 700 that constitute the system. "We were surprised by the versatility of programs we were able to design, despite being limited to six-bit inputs," says David Doty, fellow lead author and assistant professor of computer science at the University of California, Davis. "When we began experiments, we had only designed three programs. But once we started using the system, we realized just how much potential it has. It was the same excitement we felt the first time we programmed a computer, and we became intensely curious about what else these strands could do. By the end, we had designed and run a total of 21 circuits." The researchers were able to experimentally demonstrate six-bit molecular algorithms for a diverse set of tasks. In mathematics, their circuits tested inputs to assess if they were multiples of three, performed equality checks, and counted to 63. Other circuits drew "pictures" on the DNA "scarves," such as a zigzag, a double helix, and irregularly spaced diamonds. Probabilistic behaviors were also demonstrated, including random walks, as well as a clever algorithm (originally developed by computer pioneer John von Neumann) for obtaining a fair 50/50 random choice from a biased coin. Both Woods and Doty were theoretical computer scientists when beginning this research, so they had to learn a new set of "wet lab" skills that are typically more in the wheelhouse of bioengineers and biophysicists. "When engineering requires crossing disciplines, there is a significant barrier to entry," says Winfree. "Computer engineering overcame this barrier by designing machines that are reprogrammable at a high level—so today's programmers don't need to know transistor physics. Our goal in this work was to show that molecular systems similarly can be programmed at a high level, so that in the future, tomorrow's molecular programmers can unleash their creativity without having to master multiple disciplines." "Unlike previous experiments on molecules specially designed to execute a single computation, reprogramming our system to solve these different problems was as simple as choosing different test tubes to mix together," Woods says. "We were programming at the lab bench." Although DNA computers have the potential to perform more complex computations than the ones featured in the Nature paper, Winfree cautions that one should not expect them to start replacing the standard silicon microchip computers. That is not the point of this research. "These are rudimentary computations, but they have the power to teach us more about how simple molecular processes like self-assembly can encode information and carry out algorithms. Biology is proof that chemistry is inherently information-based and can store information that can direct algorithmic behavior at the molecular level," he says. ******************************************************************* References: 1. Original story scource: https://www.caltech.edu/about/news/computer-scientists-create-reprogrammable-molecular-computing-system 2. Journal reference: Damien Woods, David Doty, Cameron Myhrvold, Joy Hui, Felix Zhou, Peng Yin & Erik Winfree. Diverse and robust molecular algorithms using reprogrammable DNA self-assembly. Nature, 2019 DOI: 10.1038/s41586-019-1014-9 3. Image source: Completed DNA algorithms Credit: Winfree Lab/Caltech  Study uncovers genetic switches that control process of whole-body regeneration The ability to regenerate lost limbs or organs just like the salamander which can easily regrow its lost limb is an ability which humans have been aspiring to achieve. Moreover even if we exclude the casualties of war and only consider the casualties of numerous road accidents leading to amputation or other injuries and medical procedures requiring removal of the organ toatally or partially. In these scenarios development of the therapeutic ability to regenerate lost limbs will serve as a great boon. A team of Researchers Led by Assistant Professor of Organismic and Evolutionary Biology Mansi Srivastava and Andrew Gehrke, a post-doctoral fellow working in her lab, ,at Harvard is shedding new light on how animals pull off the feat, along the way uncovering a number of DNA switches that appear to control genes for whole-body regeneration. The study is described in a March 15 paper in the journal Science. Using three-banded panther worms to test the process, Srivastava and Gehrke, found that a section of noncoding DNA controls the activation of a “master control gene” called early growth response, or EGR. Once active, EGR controls a number of other processes by switching other genes on or off. “What we found is that this one master gene comes on [and activates] genes that are turning on during regeneration,” Gehrke said. “Basically, what’s going on is the noncoding regions are telling the coding regions to turn on or off, so a good way to think of it is as though they are switches.” For that process to work, Gehrke said, the DNA in the worms’ cells, which normally is tightly folded and compacted, has to change, making new areas available for activation. “A lot of those very tightly packed portions of the genome actually physically become more open,” he said, “because there are regulatory switches in there that have to turn genes on or off. So one of the big findings in this paper is that the genome is very dynamic and really changes during regeneration as different parts are opening and closing.” Before Gehrke and Srivastava could understand the dynamic nature of the worm’s genome, they had to assemble its sequence — no simple feat in itself. “That’s a big part of this paper,” Srivastava said. “We’re releasing the genome of this species, which is important because it’s the first from this phylum. Until now there had been no full genome sequence available.” It’s also noteworthy, she added, because the three-banded panther worm represents a new model system for studying regeneration. “Previous work on other species helped us learn many things about regeneration,” she said. “But there are some reasons to work with these new worms.” For one thing, they’re in an important phylogenetic position. “So the way they’re related to other animals … allows us to make statements about evolution.” The other reason, she said, is, “They’re really great lab rats. I collected them in the field in Bermuda a number of years ago during my postdoc, and since we’ve brought them into the lab they’re amenable to a lot more tools than some other systems.” While those tools can demonstrate the dynamic nature of the genome during regeneration — Gehrke was able to identify as many as 18,000 regions that change — what’s important, Srivastava said, is how much meaning he was able to derive from studying them. She said the results show that EGR acts like a power switch for regeneration — once it is turned on, other processes can take place, but without it, nothing happens.“We were able to decrease the activity of this gene and we found that if you don’t have EGR, nothing happens,” Srivastava said. “The animals just can’t regenerate. All those downstream genes won’t turn on, so the other switches don’t work, and the whole house goes dark, basically.” While the study reveals new information about how the process works in worms, it also may help explain why it doesn’t work in humans. “It turns out that EGR, the master gene, and the other genes that are being turned on and off downstream are present in other species, including humans,” Gehrke said. “The reason we called this gene in the worms EGR is because when you look at its sequence, it’s similar to a gene that’s already been studied in humans and other animals,” Srivastava said. “If you have human cells in a dish and stress them, whether it’s mechanically or you put toxins on them, they’ll express EGR right away.” The question is, Srivastava said, “If humans can turn on EGR, and not only turn it on, but do it when our cells are injured, why can’t we regenerate? The answer may be that if EGR is the power switch, we think the wiring is different. What EGR is talking to in human cells may be different than what it is talking to in the three-banded panther worm, and what Andrew has done with this study is come up with a way to get at this wiring. So we want to figure out what those connections are, and then apply that to other animals, including vertebrates that can only do more limited regeneration.” Going forward, Srivastava and Gehrke said they hope to investigate whether the genetic switches activated during regeneration are the same as those used during development, and to continue working to better understand the dynamic nature of the genome. “Now that we know what the switches are for regeneration, we are looking at the switches involved in development, and whether they are the same,” Srivastava said. “Do you just do development over again, or is a different process involved?” The team is also working on understanding the precise ways that EGR and other genes activate the regeneration process, both for three-banded panther worms and for other species as well. In the end, Srivastava and Gehrke said, the study highlights the value of understanding not only the genome, but all of the genome — the noncoding as well as the coding portions. “Only about 2 percent of the genome makes things like proteins,” Gehrke said. “We wanted to know: What is the other 98 percent of the genome doing during whole-body regeneration? People have known for some time that many DNA changes that cause disease are in noncoding regions … but it has been underappreciated for a process like whole-body regeneration. “I think we’ve only just scratched the surface,” he continued. “We’ve looked at some of these switches, but there’s a whole other aspect of how the genome is interacting on a larger scale, not just how pieces open and close. And all of that is important for turning genes on and off, so I think there are multiple layers of this regulatory nature.” “It’s a very natural question to look at the natural world and think, if a gecko can do this, why can’t I?” Srivastava said. “There are many species that can regenerate, and others that can’t, but it turns out if you compare genomes across all animals, most of the genes that we have are also in the three-banded panther worm so we think that some of these answers are probably not going to come from whether or not certain genes are present, but from how they are wired or networked together, and that answer can only come from the noncoding portion of the genome.” *************************************** References: 1. Original article : https://news.harvard.edu/gazette/story/2019/03/harvard-study-unlocks-a-key-to-regeneration/ Note: Content edited for style and length. 2.Regeneration timelapse Video link: https://news.harvard.edu/wp-content/uploads/2019/03/press_movie_2_Mansi_Srivastava_and_Andrew_R_Gehrke.mp4 3. Image: Dr. Srivastava in her lab along with Dr. Gehrke.  Traditionally we have been teaching and learning that evolution is a continuous process and sometimes takes millions of years to manifest its effect at the macromolecular level or at the organism level. This information give birth to one of the most sought after query, how rapidly new proteins evolve in organisms? Now recently a new study led by scientists from the University of Chicago challenged one of the classic assumptions about how new proteins evolve. Their findings are published in Nature Ecology and Evolution Now for the uninitiated ones (Proteins are the building blocks that carry out the basic functions of life. As the genes that produce them change, the proteins change as well, introducing new functionality or traits that can eventually lead to the evolution of new species.) One of the key outcomes of the research was that they were able to demonstrate that random, noncoding sections of DNA can quickly evolve to produce new proteins. These de novo, or “from scratch,” genes provide a new, unexplored way that proteins evolve and contribute to biodiversity. “Using a big genome comparison, we show that noncoding sequences can evolve into completely novel proteins. That’s a huge discovery,” said Manyuan Long, PhD, the Edna K. Papazian Distinguished Service Professor of Ecology and Evolution at UChicago and senior author of the new study. A third way for genes to evolve For decades, scientists believed that there were only two ways new genes evolved: duplication and divergence or recombination. During the normal process of replication and repair, a section of DNA gets copied and creates a duplicate version of the gene. Then, one of these copies may acquire mutations that change its functionality enough that it diverges and becomes a distinct new gene. With recombination, pieces of genetic material are reshuffled to create new combinations and new genes. However, these two methods only account for a relatively small number of proteins, given the total number of possible combinations of amino acids that comprise them. Scientists have long wondered about a third mechanism, where de novo genes could evolve from scratch. All organisms have long stretches of genetic material that do not encode proteins, sometimes up to 97 percent of the total genome. Is it possible for these noncoding sections to acquire mutations that suddenly make them functional? This has been difficult to study because it requires high-quality reference genomes from several closely related species that show both the ancestral, noncoding sequences and subsequent new genes that evolved from them. Without this clear, visible line of evolution, there’s no way to prove it’s truly a de novo gene. The supposed new genes reported previously could just be an “orphaned gene” that diverged or transferred from unrelated organisms at some point, then all traces of its predecessors disappeared. To overcome these challenges, Long’s team took advantage of 13 new genomes sequenced and annotated recently from 11 closely-related species of rice plants, including Oryza sativa, the most common food crop. He worked with groups headed by Prof. Rod Wing at the University of Arizona. Prof. Yidan Ouyang from Huazhong Agricultural University, China, also led a team that cultivated their own rice plants in Hainan, a tropical island off the southern coast of China, and harvested them for proteomics sampling. After analyzing the genomes of these plants, they detected at least 175 de novo genes. Further mass spectrometry analysis of protein activity was conducted by another group led by Prof. Siqi Liu at BGI-Shenzhen, a genome sequencing center located in Shenzhen, Guangdong, China. They found evidence that 57 percent of these genes actually translated into new proteins, including more than 300 new peptides. With this first, large dataset of authentic de novo genes, Long’s team detected a pattern in their evolution. It began with the early evolution of expression, followed by subsequent mutation into protein coding potentials for almost all de novo genes. “This makes sense given the widely observed expression of intergenic regions in various organisms,” said Li Zhang, a postdoctoral researcher at UChicago and lead author of the article. Long says that the Oryza plants are good genomes to search for de novo genes because they are relatively young—you can still see evidence of evolution in their existing genomes. “The 11 species diverged from each other only about three to four million years ago, so they are all young species,” he said. “For that reason, when we sequence the genomes, all the sequences are highly similar. They haven't accumulated multiple generations of changes, so all the previous non-coding sections are still there.” The path ahead Long and his team next want to study the new proteins to further understand their function and evolution and see if there is something unique about their structure. If de novo genes open up an unexplored path for evolution, they could reveal mechanisms for creating new and improved cellular functions. For instance, the researchers detected evidence of natural selection acting to fix insertions and deletions in the genome to generate new protein sequences, and the sequence’s evolution toward improved functions. “The new proteins may make certain functions better, or help regulate the genes better,” he said. “Each step of the way, they can bring some kind of benefit to the organism until it gradually becomes fixed in the genome.” ************************************ Original article written by Matt Wood, a senior science writer at UChicago Medicine and the Biological Sciences Division. Note: Content may be edited for style and length. Reference: 1.Original article: https://www.uchicagomedicine.org/forefront/biological-sciences-articles/2019/march/genes-that-evolve-from-scratch-expand-protein-diversity 2. Li Zhang, et. al, Rapid evolution of protein diversity by de novo origination in Oryza. Nature Ecology & Evolution, 2019; DOI: 10.1038/s41559-019-0822-5  Snakes are one group of animals which always instill an element of fear into the minds of most creatures and the fear of snake may have evolutionary links to our development as it was one of the prominent threat in the wilderness during our hunter gatherer days. However the fear deffinitely do not deter herpetologists and snake lovers to look out for an encounter with a beautiful snake as they are often motivated by the beauty and diversity of these creatures. The discovery we are talking about was made by a team of scientists and researchers led by Dr. Mark-Oliver Roedel from Berlin's Natural History Museum, in the rain forests of Southeastern Guinea and Northwestern Liberia. The team discovered a new species of stiletto snake which can stab sideways and jump a distance equal to its own body length has been discovered in West Africa. Three specimens were found by a team of scientists and were later all identified as a species previously unknown to science. The snake is from a family of vipers which have teeth protruding from the sides of their mouths, allowing them to strike prey with their venomous fangs from an unusual angle and without even opening their mouths. The group is also known as mole vipers or burrowing asps and, due to their unusual physiology, they cannot be handled as other snakes can by holding them behind the head. While most of these burrowing snakes are not venomous enough to kill a human, some are able to inflict serious tissue necrosis, which could lead to the loss of a finger or thumb. The species has been named Branch's stiletto snake or Atractaspis branchi, in honour of the South African herpetologist Prof. William Branch, a world-leading expert on African reptiles who died in February 2017. The first specimen was collected at night from a steep bank of a small rocky riverbed in a lowland in the evergreen forest of Liberia. The team's findings have been published in the open-access journal Zoosystematics and Evolution Reference: 1. Find the original article here https://www.independent.co.uk/news/science/snake-new-species-stiletto-viper-guinea-liberia-a8820841.html 2. Journal link: https://zse.pensoft.net 3. Image: A Stiletto Snake, Wikimedia |
AuthorHello! My name is Arunabha Banerjee, and I am the mind behind Biologiks. Leaning new things and teaching biology are my hobbies and passion, it is a continuous journey, and I welcome you all to join with me Archives
June 2024
Categories
All
|




 RSS Feed
RSS Feed



